Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Tsukahara, Takehiko*; Brandt, A.*; Muto, Yuki*; Nabatame, Nozomi*; Kitatsuji, Yoshihiro
Analytical Sciences, 37(12), p.1789 - 1794, 2021/12
Times Cited Count:1 Percentile:6.35(Chemistry, Analytical)We attempted to scale down a separation process of uranium (U) using the microchip column loaded with anion exchange resin to develop safety and waste-reducing separation technique. The ideal separation performance of U was obtained by the properly design of a microchannel. The concentration of U in seawater as a real-world sample could be quantified with the prepared microchip column. It indicates that the microchip column is sufficiently practical. Compared to separation of U with a general column, the column size was successfully scaled down to  1/5000.
1/5000.
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:4 Percentile:12.17(Chemistry, Analytical)no abstracts in English
Ozawa, Mayumi; Fukaya, Hiroyuki; Sato, Makoto; Kamohara, Keiko*; Suyama, Kenya; Tonoike, Kotaro; Oki, Keiichi; Umeda, Miki
Proceedings of 53rd Annual Meeting of Hot Laboratories and Remote Handling Working Group (HOTLAB 2016) (Internet), 9 Pages, 2016/11
Zhao, Y.; Yoshimura, Kimio; Shishitani, Hideyuki*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Koizumi, Satoshi*; Szekely, N.*; Radulescu, A.*; Richter, D.*; Maekawa, Yasunari
Soft Matter, 12(5), p.1567 - 1578, 2016/02
Times Cited Count:27 Percentile:80.24(Chemistry, Physical)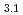 (OCOCH
(OCOCH )
)
 0.9H
0.9H O
OKozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Microporous and Mesoporous Materials, 89(1-3), p.123 - 131, 2006/03
Times Cited Count:11 Percentile:39.24(Chemistry, Applied)Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. Although LTMHSs have recentely attracted attention of researches on anion exchange and intercalation, very limited numbers of reports have been published on their synthesis, characteristics, and applications. This paper describes basic characteristics of a new LTMHS, nickel-copper hydroxide acetate. Hydrothermal Heating of an aqueous solution containing nickel acetate, copper acetate, and hydrogen peroxide to 150 C for 4h yielded a layered compound with an analytical composition of NiCu(OH)
C for 4h yielded a layered compound with an analytical composition of NiCu(OH)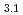 (OCOCH
(OCOCH )
) 0.9H
0.9H O. This compound does not take up Cl
O. This compound does not take up Cl and NO
and NO
 in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
 and AsO
and AsO
 . This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl
. This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl and NO
and NO
 .
.
Kozai, Naofumi; Onuki, Toshihiko; Komarneni, S.*
Journal of Materials Chemistry, 17(12), p.2993 - 2996, 2002/12
Here we report the extremely high and selective uptake of selenium oxyanions by a novel exchanger, Ni Zn
Zn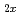 (OH)
(OH) (OCOCH
(OCOCH )
)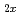 ・nH
・nH O that has brucite-type hydroxide layers with OCOCH
O that has brucite-type hydroxide layers with OCOCH anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10
anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10 ml/g with an initial Se(IV) concentration of 1x10
ml/g with an initial Se(IV) concentration of 1x10 M) in the presence of 0.1M Cl
M) in the presence of 0.1M Cl solution while the well known anionic clay, Mg
solution while the well known anionic clay, Mg Al(OH)
Al(OH) NO
NO ・nH
・nH O showed a Kd of 6.0x10
O showed a Kd of 6.0x10 ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl
ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl , 1N NO
, 1N NO
 , or 1N PO
, or 1N PO
 , while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl
, while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl solution. This novel exchanger also showed high Kd (2.6
solution. This novel exchanger also showed high Kd (2.6 10
10 ml/g at an initial Se concentration of 1
ml/g at an initial Se concentration of 1 10
10 M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
 solutions
solutionsHaba, Hiromitsu; Tsukada, Kazuaki; Asai, Masato; Goto, Shinichi*; Toyoshima, Atsushi; Nishinaka, Ichiro; Akiyama, Kazuhiko; Hirata, Masaru; Ichikawa, Shinichi; Nagame, Yuichiro; et al.
Journal of Nuclear and Radiochemical Sciences, 3(1), p.143 - 146, 2002/06
We have investigated the sorption behavior of element 104 rutherfordium (Rf) on an anion exchange resin from HCl and HNO solutions. In the HCl experiments, the distribution coefficients of Rf increase with an increase of HCl concentration from 7.0 M to 11.5 M, indicating that anionic species such as [Rf(OH)Cl
solutions. In the HCl experiments, the distribution coefficients of Rf increase with an increase of HCl concentration from 7.0 M to 11.5 M, indicating that anionic species such as [Rf(OH)Cl ]
] or [RfCl
or [RfCl ]
] are formed. This sorption behavior of Rf is typical of the group-4 elements Zr and Hf, and is quite different from that of the pseudo-homologue Th. It is also noted that the distribution coefficients decrease in the order Rf, Zr, Hf at 9.0 M HCl, which is consistent with the expected order of ionic radii. On the other hand, Rf appears to behave like Zr and Hf in 8 M HNO
are formed. This sorption behavior of Rf is typical of the group-4 elements Zr and Hf, and is quite different from that of the pseudo-homologue Th. It is also noted that the distribution coefficients decrease in the order Rf, Zr, Hf at 9.0 M HCl, which is consistent with the expected order of ionic radii. On the other hand, Rf appears to behave like Zr and Hf in 8 M HNO not like Pu and Th.
not like Pu and Th.
 -HI mixed acid solution
-HI mixed acid solutionUsuda, Shigekazu; Sakurai, Satoshi; Hirata, Masaru; Umezawa, Hirokazu
Sep. Sci. Technol., 25(11-12), p.1225 - 1237, 1990/00
Times Cited Count:1 Percentile:27.36(Chemistry, Multidisciplinary)no abstracts in English
Usuda, Shigekazu; Umezawa, Hirokazu
Journal of Radioanalytical and Nuclear Chemistry, Letters, 127(6), p.437 - 446, 1988/06
no abstracts in English
; *; ;
Journal of Radioanalytical and Nuclear Chemistry, Letters, 117(6), p.329 - 336, 1987/06
no abstracts in English
Journal of Radioanalytical and Nuclear Chemistry, 111(2), p.399 - 410, 1987/02
Times Cited Count:15 Percentile:79.68(Chemistry, Analytical)no abstracts in English
Journal of Radioanalytical and Nuclear Chemistry, 111(2), p.477 - 485, 1987/02
Times Cited Count:8 Percentile:63.44(Chemistry, Analytical)no abstracts in English
; ; *; ;
Journal of Radioanalytical and Nuclear Chemistry, 116(1), p.125 - 132, 1987/01
Times Cited Count:3 Percentile:38.12(Chemistry, Analytical)no abstracts in English
JAERI 1273, 60 Pages, 1981/12
no abstracts in English
Journal of Nuclear Science and Technology, 6(1), p.12 - 19, 1969/00
no abstracts in English
Nihon Genshiryoku Gakkai-Shi, 4(6), p.386 - 389, 1962/00
no abstracts in English
; ;
Bulletin of the Chemical Society of Japan, 34(7), p.952 - 955, 1961/00
Times Cited Count:49no abstracts in English


 Th(UX
Th(UX ) by anion exchange fromnitric acid-alcohol mixed solution of uranyl nitrate
) by anion exchange fromnitric acid-alcohol mixed solution of uranyl nitrateBulletin of the Chemical Society of Japan, 34(8), p.1198 - 1198, 1961/00
Times Cited Count:8no abstracts in English
Maekawa, Yasunari
no journal, ,
Yoshimura, Kimio; Koshikawa, Hiroshi; Yamaki, Tetsuya; Shishitani, Hideyuki*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Maekawa, Yasunari
no journal, ,
Since anion-conducting electrolyte membrane fuel cells (AEMFCs) such as direct hydrazine fuel cells operated under high temperature and highly basic condition, it is required to develop alkaline durable AEMs. In this work, we synthesized AEM with poly 2-methyl-1-vinylimidazolium grafts by radiation-induced graft coplymerizaiton to protect 2-position of imidazolium ring from alkaline hydroxide ion attack. The AEM with an ion-exchange capacity (IEC) of 1.80 mmol g exhibited ionic conductivity of 123 mS cm
exhibited ionic conductivity of 123 mS cm at 60
at 60 C. The durability of the membrane was evaluated. The excellent long-term alkaline stability of the prepared AEM was confirmed in 1M KOH at 80
C. The durability of the membrane was evaluated. The excellent long-term alkaline stability of the prepared AEM was confirmed in 1M KOH at 80 C. Therefore, the protection of 2-position by methyl group suppresses ring opening degradation reactions, resulting in the improvement of the alkaline durability of imidazolium-type AEMs.
C. Therefore, the protection of 2-position by methyl group suppresses ring opening degradation reactions, resulting in the improvement of the alkaline durability of imidazolium-type AEMs.